 To read other articles in this series, click here.
To read other articles in this series, click here.
Let’s look at how much energy the oceans can store compared to the energy storage of the atmosphere.
One way to describe the amount of energy that something can store is called “specific heat.” This is essentially the amount of energy required to heat up a mass of a material by a certain temperature. In our case, we’ll use 1 kg heated by by 1 degree Celsius (1.8° F) because those are the international standards.
The specific heat of air is about 1158 J/(kg*C) while the specific heat of seawater is about 3850 J/(kg*C), where a Joule is a standard measurement of energy. We can see that air has a specific heat a little more than 3x smaller than that of water. But we know from our day-to-day experience that water is a lot denser than air is, and that will matter a great deal to our calculations. (For reference, one Joule is about the amount of energy you need to expend to lift one pound 9 inches.)
While we could go through a huge amount of geometry to estimate how much air and seawater there is on the Earth, but there’s an easier way – use the measurements of experts. for example, this paper calculated that the total mass of the atmosphere is about 5.14 x 1018 kg, while the National Oceanic and Atmospheric Administration (NOAA) has calculated that the total volume of the world’s oceans is about 1.34 x 10^18 m3. In order to get the total mass of the world’s oceans we need an estimate of the density of seawater, which I found at this MIT link – 1027 kg/m3 (other sources have similar values).
Using this, we can multiply the mass of the atmosphere times the specific heat of the air to calculate what the total heat capacity of the atmosphere is:
(Eqn. 1)
In other words, it takes about 5.95 x 1021 Joules to raise the temperature of the atmosphere one degree Celsius.
For ocean we need to add one step – multiplying the volume of the water by its density to get the total mass of the ocean
(Eqn. 2)
This shows that the heat capacity of the oceans is about 1000x larger than the heat capacity of the Earth’s atmosphere.
So why do we care? First, it helps to explain why we care about El Nino and La Nina cycles in the Pacific Ocean. If you’re unfamiliar with the terms, La Nina is a massive upwelling of cold water in the Pacific that, because ocean water has a much higher heat capacity than air, cools off the entire planet and affects weather patterns. El Nino is a massive pool of hot water in the Pacific that does the opposite – it dumps heat stored in the ocean back into the atmosphere, warming the globe and affecting weather patterns. Nearly all the energy absorbed by the Pacific Ocean during La Nina periods will eventually be emitted back into the atmosphere during El Nino periods.
Second, the heat capacity of the world’s oceans helps to explain why scientists are so interested in how much energy has been stored in the ocean. Since total ocean heat capacity is about 1000x greater than total atmosphere, it means that a barely measurable temperature increase in the ocean (1/1000th of a degree C) could drive a massive spike in global air temperature (1 degree C).

The difference between measured global surface temperature from various sources and the temperatures adjusted to remove the influence of El Nino, volcanoes, and the solar cycle. Note that the massive 1997/1998 El Nino spike is nearly completely the result of ocean El Nino dumping stored energy into the atmosphere. (Image Credit: Skeptical Science)
Lastly, we care because it demonstrates just why the average global temperature hasn’t been warming as fast over the last several years. We’ve had more La Nina cycles since 1998 than we’ve had El Nino cycles, and that means the Pacific ocean is storing more energy.
The problem with this, however, is that it means that energy is going to come back OUT of the ocean again eventually. And when (not if) that happens next, the average global temperature will spike.
Categories: Environment/Nature, Science/Technology, Uncategorized




Well fuck me, I’m going to go have a drink now…and maybe watch a marathon of Independence Day, The Day After Tomorrow and 2012. But good info… 🙂
This is depressing, Brian.
Why depressing, Denny? “Worrying” I get, but you’ve got me confused.
I think that you should re-do your calculations using just the volume of the troposphere, not the total atmosphere. Also, are you saying that because of the disparity in total heat energy that the oceans can raise the air temperature above the temperature of the water? Last I checked, heat energy moved from higher temperature bodies to lower temperature bodies.
Clyde Spencer said “I think that you should re-do your calculations using just the volume of the troposphere, not the total atmosphere.” – OK. A quick Google search indicates that 75-80% of the mass of the atmosphere is in the troposphere, so the calculation drops from 5.95 x 1021 J/C to 4.46 x 1021 J/C. That changes the number from 890x to 1190x. Still about 1000x, so that has no impact on my conclusions.
“Also, are you saying that because of the disparity in total heat energy that the oceans can raise the air temperature above the temperature of the water? Last I checked, heat energy moved from higher temperature bodies to lower temperature bodies.” – I really ought to write a post debunking the “AGW breaks the 2nd Law of Thermodynamics” claims one of these days, because it’s based on a common misunderstanding.
Imagine two kids playing with water. One has a hose, the other has a small squirt gun. Which one gets wetter? The one without the hose, obviously, because there’s a lot more water coming out of the hose. But does that mean the kid who’s holding the hose never gets wet from the squirt gun? Of course not. Both kids get wet, but one kid gets a lot wetter.
Heat flow is very much like this analogy. The net flow of energy is from the hotter body to the cooler one, but there is a small amount of energy that flows (mostly as IR photons) from cold to hot. The hotter body will actually be slightly hotter due to the energy flow from the cold body, but the cold body will still be the one that warms up.
Put another way, if you put a spacecraft between the Earth and the sun, do you see the Earth? Of course you do. Now take that spacecraft away and what happens to those photons? They’re absorbed by sun. And so a tiny amount of energy flows from the cold Earth to the hot sun, even as the net energy flow is from the hot sun to the cold earth.
“The hotter body will actually be slightly hotter due to the energy flow from the cold body, but the cold body will still be the one that warms up.”
Simply not true. The hotter body will only cool slower if there is a cold body (above background temperature) present. Never ever will a cold body make a hot body even hotter. Not even a tiny little bit.
Clear Sky, your statement is based on a strictly classical understanding of thermodynamics. When you start including quantum effects (namely the fact that energy is emitted in discrete amounts and is emitted at random, discrete points in time), we end up needing to update our understanding a bit. To see what I mean, let’s consider a simplified two particle system and let’s abstract the temperatures of the particles to discrete, whole numbers.
Particle A has a temperature of 8 while Particle B has a temperature of 7. Per classical mechanics, energy flows exclusively from particle A to Particle B until both particles have a temperature of 7.5 and no energy flows between them. But let’s add quantum mechanics to the mix. Now, both particles can only have temperatures of 0, 1, 2, 3, etc. and energy can only be exchanged between the particles in packets of 1, 2, 3, etc. In this case, if Particle A emits 1 energy to Particle B, Particle A’s temperature drops to 7 and Particle B’s temperature increases to a temperature of 8.
But what happens if Particle A emits 1 energy at the same time that Particle B emits 2 energy? In this case, Particle A’s temperature drops from 8 to 7 at the same time that Particle B’s temperature drops from 7 to 5. The total energy in the system is still the same, but now there’s three packets of energy in transit between the two particles. When Particle B absorbs the 1 energy emitted by Particle A, it heats up from 5 to 6. When Particle A absorbs the 2 energy emitted by Particle B, it heats up from 7 to 9. In essence, Particle B warmed up Particle A.
Now, the fact that hotter objects radiate energy faster than colder objects do means that Particle A will radiate its extra energy back to Particle B faster than Particle B (which is now 3 temperature colder than Particle A, rather than 1 temperature it started at) will radiate its energy. So the net energy flow will be from Particle A to Particle B. This also means that the example I gave (cooler Particle B radiating two energy to Particle A’s radiating one energy) is less probable than the two particles radiating one energy back and forth to each other, but that doesn’t make it impossible.
We can see this more clearly if we reduce the simplification a little and say that the two particles are actually an infinite planes of material with one side facing each other and the other side facing an infinite cold background. In this case, each sheet has a 50/50 chance of radiating 1 energy at the other sheet or radiating that energy into the cold background. Let’s again say that Sheet A has a temperature of 8 while Sheet B has a temperature of 7. But this time, Sheet B radiates 2 energy (going from 7 to 5) at Sheet A while Sheet A radiates 1 energy into the background (going from 8 to 7). When the two energy emitted by Sheet B are absorbed by Sheet A, its temperature increases from 7 to 9, effectively resulting in a cold object temporarily increasing the temperature of a hob object. Conservation of energy is maintained because the system now includes the cold background in addition to the two sheets. And entropy stays the same or increases as it must (one energy is lost to the background). And the temporarily hotter Sheet A will now radiate it’s extra energy more quickly, resulting in a net transfer of energy from the hotter sheet to the colder sheet (and ultimately to the cold background).
This works for the Sun/Earth system too. Electromagnetic radiation absorbed by the Earth and re-emitted back to the sun (mostly as infrared photons) cool the Earth when they’re emitted, and when they’re absorbed by the Sun’s surface, those photons temporarily warm the particle(s) that absorbed the photons. The extra energy will be re-radiated or conducted away almost instantly, but for a fraction of a nanosecond, a tiny piece of the Sun’s 5000K surface becomes 5000.000…01K instead because of infrared photons emitted by the Earth at 300K. The net, classical thermodynamic effect is that the Sun cools slower than it would have without the presence of the Earth.
Well ain’t that a kick in the family Joules.
But thank you for the post. This is one (of many actually) subject that keeps me pretty confused. You have just countered most of the anti-climate change arguments I have been seeing lately that cite the last 15 years of temperatures to say it ain’t so.
Onward through the fog.
Thanks for the in depth explanation! True, that the average global temperature isnt’ rising as fast as many would think; however, there are still things that we need to do globally to help Mother Nature out!
CO2 is Mother Nature’s preferred food source. The probability of us falling into another ice age is infinitely higher than continued warming, in fact is is an almost certainty. People starve, freeze and go to war during ice ages (note multiple Revolutions during the Little Ice Age, including our own). [SNIP]
[ADMIN: This comment is off-topic for this post. Any further comments of this type should be taken to other posts where they are more relevant.]
Actually this is very very good news. CO2 absorbs between 13µ and 18µ. Those wavelengths don’t penetrate or warm the oceans. Given the ocean contains 1,000x the heat of the atmosphere, there is no way for the atmosphere, let alone the spectrum CO2 absorbs to warm the oceans. The oceans are warming because more visible light is reaching the oceans, and that has nothing to do with CO2. The fact that the oceans are warming is a smoking gun that CO2 isn’t the cause, unless you can explain how 2.2W/M^2 trapped in the atmosphere can warm the oceans. Simply put, there isn’t enough heat in the atmosphere, and changing CO2 from 280 to 400 doesn’t change that calculation much. BTW, the sun warming the oceans not only released CO2 it releases H2O. Add H2O to any MODTRAN calculation and you sell see CO2 is irrelevant.
CO2isLife – there is so much wrong with your comment that it’s hard to know where to start correcting it.
First, you’ve got the absorption of water backwards – it’s a weak absorber in the visible wavelengths but strong in both UV and IR.


Second, carbon dioxide (CO2) absorbs (and re-emits) at 2, 3, between 4 and 5, and between about 13 and 17 microns (red line in my MODTRAN-derived graph below). If you compare that to the graph above, you’ll notice that liquid water has a strong absorption band right at 2 microns, another at 3 microns, it’s lower than a peak but still strong from 4 to 5 microns, and the last high point before absorption starts slowly tailing off is between 10 and 20 microns. In other words, you can see from the graphs that CO2‘s absorption/emission bands are right where liquid water’s are.
Third, while it’s true that CO2 and water vapor absorb a lot of the IR before it reaches the ocean, that doesn’t prevent the atmosphere from warming the oceans directly. Any photons that are absorbed will be re-emitted, usually at the same photon energy (there are exceptions – two photons with half the energy could be emitted instead, for example, or the energy could be transferred to another molecule via a collision), and those photons can warm the ocean just fine. Energy absorbed in the atmosphere can still reach the surface – it just reaches the surface less efficiently, and the greater the absorption, the lower the efficiency.
This lower efficiency of transmission is the same mechanism that explains how the ocean warms up, in fact. Lowering the rate of energy loss from the oceans while keeping the energy input the same must increase the temperature of the oceans. Anything else results in breaking conservation of energy.
You can model this as a simple electrical circuit, in fact. Treat the heat flow into the ocean is a constant current source and the thermal loss out of the ocean through the atmosphere is a resistor. The temperature of the ocean is analogous to the voltage at the node where the current source meets the resistor. Voltage equals current times resistance, or in this case, temperature equals heat flow times thermal resistance. Increase the resistance, increase the temperature. It’s pretty much that simple.
Finally, you’re making the “CO2/water vapor is saturated” argument, but like most everything else in your comment, it’s wrong. Explaining why is beyond the scope of a comment, but I’ll refer you to the Skeptical Science explanation (http://www.skepticalscience.com/saturated-co2-effect.htm) and the explanation at RealClimate instead (http://www.realclimate.org/index.php/archives/2007/06/a-saturated-gassy-argument/).
Brian, water is a weak absorber of visible light, but the ocean is very deep, so deep that it absorbs all of it.
Also, your absorption bands of CO2 at 2, 3 and 4 microns is not relevant because the earth does not radiate in those wavelengths so it has nothing to do with the greenhouse effect.
You are right that H2O is a weak absorber of Visible light that is why it penetrates the oceans. That energy doesn’t suddenly disappear, it is transformed to heat. IR is absorbed in the microlayer of the oceans and most likely causes cooling due to evaporation. Care to provide any empirical evidence that shining IR between 13µ and 18µ can warm water? Either way, the oceans hole 1,000x the heat of the atmosphere, CO2 is the atmosphere only traps 1/10th of that heat. That means heat in the atmosphere trapped by CO2 is 1/10,000 that of the oceans. There simply isn’t enough heat trapped by CO2 to make a difference. Feel free to prove me wrong…right after you debunk the 1st Law of Thermodynamics.
That comment demonstrates an ignorance of the AGW/GHG effect. The GHG results from CO2 trapping IR being emitted from the earth. The earth emits around 10µ The only wavelengths that CO2 absorbs that is even remotely related to that are 13µ to 18µ. CO2 does absorb spikes at 2.7µ and 4.3µ but those are irrelevant. Spectrum of Venus? Really? 98% vs 0.0004% CO2, try Mars, that is a better example, and note the huge difference between day and night temperatures. Before I ever used Venus to make my case, I would admit defeat. That is a totally misleading example.
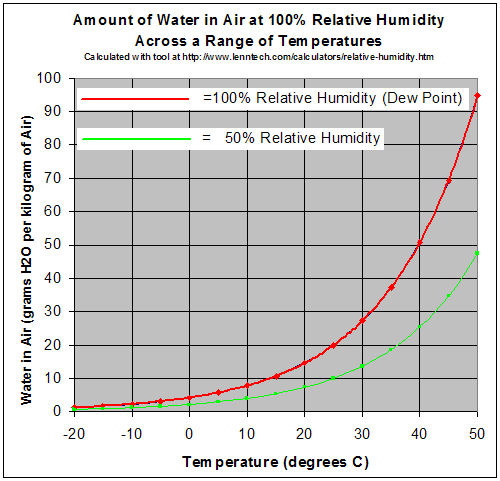
Maybe you aren’t familiar with the meaning of this chart.

No argument there, where there is H2O in the atmosphere there is warmth. Same can;t be said about CO2. Sleep naked in a rain forest and you are comfortable, sleep naked in a very dry desert and you will freeze to death. A natural experiment controlling for H2O in the atmosphere to prove CO2 doesn’t trap much heat.
That is easily proven in a lab. Please provide the empirical evidence that IR between 13µ and 18µ at 3W/M^2 can warm a bucket of water. Isolate the wavelengths and intensity of CO2’s contribution to the GHG effect and prove it can war water. Just show me the evidence and I’ll be convinced. Once again, look at the intensity of incoming visible light and outgoing IR. It take a whole lot of radiation to warm the oceans, there simply isn’t enough between 18µ at 3W/M^2 to do that.

Once again, visible light that penetrates the oceans doesn’t just disappear. That energy is converted to heat. You can’t warm the deeper oceans if you never reach the deeper oceans. IR never reaches the deeper oceans. Your comment proves my point.
I’m not wrong then, the calculator in the NASA designed MODTRAN program is wrong, and I doubt that. Simply use that program to see how irrelevant CO2 is when H2O is added to the mix. H2O traps over 70x the heat of CO2 is some cases. Just plug in the conditions emerging from the last ice age, 180 ppm CO2 and 0% H2O. Increase the CO2 to 200ppm, and change the H2O from 0 to 1. H2O traps 70x more heat than CO2, and that increase CO2 and H2O wasn’t due to man and his SUV, it was due to the sun.
Thanks for helping me prove the AGW theory is pure nonsense, your failed attempt to defend it pretty much proves my point. Venus? Really? That is the best you can do?
CO2isLife – First, let me correct a misconception you have about my previous comment. The image with the CO2 absorption spectrum overlaid on the black body spectrum of Venus’ surface temperature was not intended to imply anything about Venus. It was a convenient image I had generated using MODTRAN that had the CO2 spectrum on it, nothing more. It was a repurposed image from a series of posts debunking claims that Venus’ surface temperature wasn’t due to the greenhouse effect that I wrote years ago (here if you’re interested). I should have made that more clear.
Now, on to the rest of your comment.
“That energy doesn’t suddenly disappear, it is transformed to heat. IR is absorbed in the microlayer of the oceans and most likely causes cooling due to evaporation.” Of course the energy doesn’t just disappear. I didn’t say it did. It would be absorbed by dissolved minerals, suspended solids, etc. The ocean gets dark if you go deep enough, after all.
As for whether or not IR absorption causes cooling, that’s a possibility, but again we can do an experiment to prove it one way or another. Funny, though, several people already have done simple experiments that get to some of this.
“Care to provide any empirical evidence that shining IR between 13µ and 18µ can warm water?” Roy Spencer did this experiment and showed that warm water partially shielded with a high emissivity paint-coated aluminum panel cooled slower than water that was unshielded. Not a perfect experiment to be sure, but good enough to demonstrate that IR emissions were partially blocked. And since cooling and heating are physically similar (the emissivity of a material at a given wavelength is equal to its absorptivity at that same wavelength, per quantum mechanics), that means the reduction in cooling shows that water can absorb IR too.
ScottishSkeptic did a similar experiment and published his results on July 1, 2015. He used a grill set very low for 5 minutes and found that the water had warmed by about 30 °C. He also found that there might have been a hot surface layer and/or reflected IR when he measured the water with his IR thermometer. Now, doing this properly with good thermal isolation, filtering IR for the wavelengths of interest, controlling for evaporation, etc. would require a well stocked lab, which is something I don’t have. But these two basic experiments demonstrate that IR can absolutely warm water.
And fundamentally, any wavelength that is absorbed adds to the energy of the material that absorbed it. That energy can manifest in a multitude of ways, including photon emission, movement, and vibration, but the fact that the energy was increased cannot be disputed. I’m sure that some amount of IR absorbed by liquid water does, in fact, create evaporation. But given it takes five photons at a wavelength of 13 microns to give a single liquid water molecule enough energy to exceed its enthalpy of vaporization (and that’s leaving it in place – more energy is required to move it away from the water’s surface), it’s not at all clear that it would cause cooling. The chance that five photons all hit the same water molecule at the same time with just the right energy is going to be pretty small.
I have no idea what you mean about the 1st law of thermodynamics (change in internal energy equals heat added to the system minus work done by the system – see Hyperphysics). I think you mean the 2nd law, but rather than address this point now, I’ll simply wait until you can clarify.
“That comment demonstrates an ignorance of the AGW/GHG effect. The GHG results from CO2 trapping IR being emitted from the earth.” My response to your first comment was summarized with the following:
Where in that paragraph did I say anything about what direction the energy was coming from, whether it was solar irradiance or terrestrial emission? I can’t find it anywhere, so please don’t imply I said things that I didn’t actually say.
“You can’t warm the deeper oceans if you never reach the deeper oceans. IR never reaches the deeper oceans.” So what? IR doesn’t need to reach the deeper ocean for IR to be one of the causes of deep ocean warming. Neither does visible light. Wave action mixes the upper several meters (10-200 meters – this book chapter hosted by Judy Curry found mixing to depths of up to ~80 meters over a 5 day period), while the Meridonal Overturning Circulation moves tremendous amounts of heat from the tropics to the poles. Smaller currents and eddies can do the same, but over smaller areas.
“I’m not wrong then, the calculator in the NASA designed MODTRAN program is wrong, and I doubt that.” If you’d bother to check either of the links I referenced, then you’d know that I wasn’t disagreeing that the wavelengths are saturated. They are. Where you’re wrong is that their being saturated matters, because it doesn’t. Seriously – read the links and you’ll learn that a) the sidebands and fine structure absorption regions aren’t saturated and will get more so as more CO2 is added; b) adding more CO2 increases the backradiated IR from the atmosphere (because photons are emitted in a spherical volume regardless of what direction they were absorbed from) and that additional energy on the surface/in the atmosphere necessarily increases the temperature; and c) adding more CO2 to the atmosphere boosts the altitude at which heat can be lost to space, and since higher altitudes are cooler, the radiation efficiency decreases (it’s proportional to T4) and so the temperature of the surface increases to compensate.
All that said, I realized writing this response that you appear to be suffering from a fundamental misunderstanding, and that I was arguing circles around it instead of addressing it head on. The heat capacity of the atmosphere does not matter for global warming, at least not as how it relates to the temperature of the ocean. What matters is the increase in the effective thermal resistance of the atmosphere (I say “effective” because thermal resistance is a conduction concept, while the atmosphere is dominated by convection in the lower atmosphere, radiation in the upper atmosphere, and a mix of the two in the middle). Adding CO2 increases the effective thermal resistance of the atmosphere. If the amount of energy being lost from the surface is the same, then the temperature of the surface has to increase as a result of that increasing resistance. If this is something that you don’t understand, then I recommend you refresh your understanding of thermodynamics. If it’s something you understand but reject for some reason, then I’m not sure there’s anything I can do for you.
Great, you understand MODTRAN. Here is the most relevant example I can think of. CO2 180ppm, Subarctic Winter, 0% H2O, 70km, no clouds or rain . Those are the ice age conditions. Then the earth warms due to something other than CO2, there is no plausible mechanism by which CO2 can lead warming coming out of an ice age, nor is there a mechanism by which CO2 would decrease to lead the earth into an ice age. Huge unanswered questions for this “settled” science. Now change the settings to 200ppm CO2, Trapped heat goes from 215.373 W/m2 to 215.121 W/m2, whopping 0.252 W/M^2. Now change it to MidLatitude Summer 180ppm, and you get 344.144 W/m2 change it to 200ppm and you get 343.83 W/m2, or a whopping 0.314 W/M^2. Clearly CO2 doesn’t mean diddly in trapping heat, and that is a best case scenario for CO2. Now simply add the default H20 at 200ppm, and the heat trapped changes to 283.856 W/m2, or a change of 60 W/M^2, or 120x the impact of both periods of CO2. Clearly CO2 is a joke, H2O dominates the GHG effect. That is also demonstrated how temperature follows H2O in the atmosphere.
You are avoiding the elephant in the room. The Oceans are warming. IR between 13µ and 18µ being radiated by the atmosphere simply doesn’t have the energy to warm the oceans. Do the math. The atmosphere has 1/1000 the energy of the atmopshere. CO2 traps 10% of the spectrum AT THE COLD/LOW ENERGY END consistent with a black body of -50°C and -11°C. That means CO2 is responsible for at most 1/10,000 the energy of the ocean. Even is the oceans did absorb those wave lengths it wouldn’t mean diddly. (I’m assuming CO2 is fully responsible for change in W/M^2). Show me any experiment where IR between 13µ and 18µ at 4W/M^2 can warm a bucket of water. Just show me the results of that experiment.
Once again, you are avoiding the elephant in the room. That experiment didn’t even try to replicate what would prove the point, and it didn’t control for H2O. Once again, this is a “settled” science and you can’t even point to the most basic experiments needed to prove the point. Models don’t prove anything, and that is all the AGW theory has. This is pretty simple. Take a bucket of water and put it in a dry container with 0% H2O and 400ppm. Shine IR between 13µ and 18µ at 4W/M^2 on the water. Measure the temperature. If this would warm water it would be demonstrated in every high school science class, instead they watch An Inconvenient Truth.
That is and isn’t true, causing surface evaporation cools the water beneath it, much like sweat cools the body beneath it. Once again, this isn’t a difficult experiment that doesn’t seem to have been tested. We know the wavelengths, we know the CO2 concentration, we know the material to warm, and we know the W/M^2. Don’t these climate experts know how to run a controlled experiment? If this is a settled science, they don’t know what it takes to settle a science. Simply saying it is settled doesn’t make it so.
OK, I will give you that, assume 100% of the energy trapped by CO2 is absorbed by the ocean. Do the math, can that amount of energy warm the vast oceans by 0.4°C since 1978? No way in Hades, do the math.

The surface temperatures are warming, those depths reached by visible light. Record daytime temperatures have nothing to do with CO2, yet provide evidence that more warming sunlight is reaching the oceans. If CO2 was causing any warming its signal would be a narrowing of the temperatures between day and night in dry deserts. That isn’t happening. Care to provide the evidence that the deserts are trapping more heat in its CO2 rich H2O void atmosphere? You can also use Antarctica to control for CO2. It hasn’t warmed in over 50 years.

Games on, gotta go. Show me any experiments and you will convince me. This is the only “science” I know of that doesn’t even do the most basic experiments to prove the theory. It is all models, opinions, consensus, and politics.
BTW, 600 million years, CO2 as high as 7000ppm, and never did CO2 cause catastrophic warming.

OK, now this is just getting silly.
Let’s use your numbers from below – 0.252 W/m2 for a 20 ppm increase in CO2 (there’s no justification for using such a small increase, when the actual increase from an ice age to an interglacial is more like 100 ppm, or from ~180 to ~280 ppm, but that doesn’t actually matter for my math). The heat capacity of water is 1158 J/(kg*C), from above. Conveniently enough, °C and Kelvin are (K) on the same scale, so the heat capacity for water is also 1158 J/(kg*K). A Watt is defined as 1 Joule per second, so we can calculate how long it would take for 0.25 W/m2 to heat a square meter of water.
Now, we’ll also need to generate a kg of water. There are 1000 kg of water in a cubic meter, so we can determine the mass of 1 m2 area by a 1 m deep as 1000 kg. To raise that much water 1 K in temperature takes 1,158,000 (1.158 x 106 in scientific notation) joules, by simply multiplication. So if we inject 0.25 W of energy into that mass, we can calculate how many seconds that takes to raise the temperature 1 K. Divide 1.158 x 106 J/(m3K) by 0.252 J/s gives us 4.595 x 106 s/(m3K), and we multiply that by 1 K to get how many seconds it takes for 0.25 W/m2 to raise the temperature of 1 cubic meter of water, or 4.595 x 106 s/m3.
We can divide 4.595 x 106 s/ m3 by 86,400 seconds per day and we get 53.2 days. If we want to say that the sea surface temperature is the top 10 meters, then with a simplifying assumption that the sea surface layer is well mixed (which, conveniently enough, is what defines the sea surface layer), the number of days increases from 53.2 to 532, or about a year and a half. In other words, your own unjustified 20 ppm decrease in energy lost from the Earth’s surface could warm the entire surface layer of the global ocean by 2.5x what’s been observed in 5% of the times it actually took. And if we use your number for the tropics instead, it would take about 42.7 days for 1 m3 or 427 days for a 10 meter column of water.
We can turn this calculation around and determine what power would be required to raise the sea surface temperature by 0.4 K over the course of 30 years. 30 years is 10,958 days (including leap years) or 9.467 x 108 seconds. Since MODTRAN gives us values in W/m2, we can again do the calculation for 1 cubic meter and then modify linearly. To raise 1 m3 0.4 K takes 463200 J total. Dividing this by the number of seconds in 30 years gives us the heat flux required. 463200 J/9.467 x 108 s is 0.00049 W/m2. The heat flux required for a 10 m deep column of water is simply 10x that number, or 0.0049 W/m2.
Let’s look at this a third way. In 1981, the annual mean CO2 was 340.10 ppm according to ESRL (ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_annmean_mlo.txt). In 2010, 30 years later, the CO2 level in the atmosphere had risen to 389.85 ppm. If we leave the MODTRAN model at the U. of Chicago site above in its default state except we change the CO2 to 340, we find that the upward IR heat flux at 70 km in the tropical atmosphere with no clouds or rain is 290.042 W/m2. If we boost the CO2 to 390, the heat flux decreases to 289.414, for a delta of 0.628 W/m2. That kind of heat flux change would heat up the top 10 meters of the ocean 0.4 K in less than 3 months. Clearly there are ocean-atmosphere interactions that are keeping this from happening.
But these calculations blast your so-called elephant in the room into small, juicy pieces. Heat flux changes due to CO2 are absolutely capable of directly raising the ocean’s temperature by what was measured between 1980 and 2010.
Finally, as for your comment on the CO2 levels back in the Cambrian, why you think that matters is beyond me. Multicellular life had just formed, there were almost no lifeforms living outside the ocean that weren’t single-celled. Terrestrial plants hadn’t even evolved yet. And solar luminosity was something like 3% less –a whopping 40 W/m2 – than it is now. How you think that has any bearing on changes in the last million years, never mind the last 100,000 or so, is beyond me.
OK, climate “science” claims to be a real science. Here is the most basic proof you would need.
1) Ocean Temperatures have increased from 0.4°C over 30 years.
2) Specific heat of water see article above.
3) Δ W/M^2 from CO2 increasing from 330 to 440 ppm under “Tropical” “2mm drizzle, 1km, default H20 is 0.00 W/M^2. The reason I used 1k and tropical and light drizzle is to replicate the extremely humid atmosphere immediately over the oceans. CO2 is 100% negated by H20 over 70% of the globe. Any impact of CO2 would be revealed higher in the atmosphere when CO2 precipitates out. Measurements have demonstrated that there is no upper tropo lower strat “hotspot.”
4) The volume of the oceans is 1.368,569 x10^21 litres
5) Exposure time 24 hrs/day 365.25 days/yr 30 years
There is no way a marginal energy contribution of 0.00 W/M^2 can warm the oceans. MODTRAN proves this AGW theory is pure nonsense.
If climate science is a real science they would follow the Scientific Method. The Null Hypothesis is “Man does not cause climate change.” The proof would be to demonstrate that the variation in temperature over the past 50 and 150 years differs at a statistically significant 95% confidence level when compared to the entire Holocene. That, once again, the starting point of any real science. Why don’t we test that in High School Science classes? Because when you test any ice core data set out there you will find the last 50 and 150 years are well within the norm. That is why students watch An Inconvenient Truth instead of testing this nonsense.
Lastly, we will spend trillions of dollars to reduce CO2. The human and societal costs are astronomical. Wind and solar have 0% chance of ever making a significant contribution to our energy needs. We will never power our homes at night with solar, and our jets wind power. GE doesn’t use wind to power their turbine factories and Solyndra didn’t use solar to power their solar plant. Do you honestly think anything we can do will significantly alter the trend is CO2? How many schools, hospitals, drugs, roads, bridges, social services, water plants, sewers etc etc won’t be built/delivered because we want to fight this Quixotic battle? Also, what happens if we rapidly slip into another Little Ice Age? We will be completely unprepared thanks to this nonsensical folly. What happens if you’re wrong? I have 600 million years of geologic history proving you wrong, and 800k years proving a coming ice age is an almost certainty.
There’s no need to answer your ongoing MODTRAN nonsense in this comment too since I dealt with it once already. And you seem to be under the false impression that scientists have never examined the null hypothesis, when they started with that null and rejected it literally decades ago. Go back and study the history of the last 180 years of scientific progress on understanding climate change, and read the original papers by Callendar and other early researchers who were sure they were wrong until the data convinced them otherwise. Then augment your economics training with more physics (optics, thermodynamics, quantum mechanics, quantum optics, and quantum thermodynamics), geology (deep geologic time), solar astronomy, biology (how life handles different carbon isotopes would be a good starting point), and chemistry (atmospheric and marine chemistry as well as geochemistry focusing on CO2 weathering and natural sequestration) than you have right now. Come back from your ego trip and stop assuming that your layperson’s understanding of climate is somehow deeper than the understanding of people who have lived and breathed climate for decades. Usually they’ve made all these same screw-ups already (and a whole lot more that you haven’t even thought of yet) and can help you not make them yourself, if you’re wise enough to listen. Then come back to talk to me.
Because right now it’s become obvious to me that you don’t know a fraction of what you need to in order to even recognize how little you actually know.
Back that claim up, show me the ice core data that shows the past 50 and 150 years of temperature variation is 2 std deviation outside the norm for the Holocene temperature variation. Just show me the evidence. Here, I will show you how easy it is to prove me wrong.
CO2isLife, here is an ice core data set that if you test it you will find that the current temperature variation is statistically different from the Holocene. Here is the link to the data.
https://www.ncdc.noaa.gov/data-access/paleoclimatology-data/datasets
I provided you the link to all the data sets, just show me one that I can test that make the scientific case for AGW, just one. Show me the evidence. It is that easy. You make a lot of assumptions and declarations, but where is the empirical evidence? I’ve tested a lot of ice cores, and have yet to find one that rejects the Null.
CO2isLife – “I provided you the link to all the data sets, just show me one that I can test that make the scientific case for AGW, just one. Show me the evidence. It is that easy.”
OK. Gee, here’s the EPICA Dome C data from the NCDC, the very first ice core I looked at: ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/epica_domec/edc-co2.txt
Here’s a plot of the last 12,000 years of ice core data (truncated at depth 438.85) with the 2014 CO2 data (398.61 ppm) appended as time=0, as well as a linear fit and the equation for that fit.
The standard deviation of the data excluding the 2014 datapoint is 8.87. Subtracting the most recent CO2 value from the EPICA core (280.4) from the 2014 data point (398.6) gives a difference of 118.2 ppm. If we divide that by 8.87 we find that the modern CO2 level is different from the previous value by over 13 standard deviations. That’s 11 more deviations than you asked for.
Here’s my simple Excel file that I whipped up to do this calculation too. Writing the words for this comment took longer than doing the math did.
There’s your evidence. Just the one core you asked for. Given how quick it was to do this, I’d guess you’re doing something very, very wrong with your calculations but either don’t understand the math and/or science well enough to recognize your mistake or you’re so biased that you won’t see the errors staring you in the face.
Nice try, change in CO2 isn’t climate change, nor is it warming. The theory is ΔCO2 results in a ΔC° that is the theory. Show me any data set that shows the ΔC° over Δ 50 and 150 years is statistically different to the 95% confidence level when compared to the entire Holocene. The very fact that you chose to use CO2 instead of temperature is very telling, but please, show me the evidence. Show me the science, not the sophistry.
I chose to analyze CO2 because I misread your comment and analyzed the wrong thing. My apologies.
Now, as for analyzing the ice core data for temperature instead of for CO2, here are my conclusions. I again used the EPICA Dome C core, but this time I used the deuterium data from this file: http://www1.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/epica_domec/edc3deuttemp2007.txt
First, if a I perform a naïve analysis of the data for the last 12,000 years or so (down to depth 371.25) and simply look at the standard deviation, I calculate that each sigma is about 0.9 °C. However, this doesn’t take into account the trend. If we assume the trend is linear and then subtract out the trend, we find the sigma drops to 0.86 °C. Not a huge improvement, but then again the trend is clearly not linear either. However, some playing around with various polynomials (up to order 6, which is as high as Excel can go easily) and logarithmic fits doesn’t improve the sigma past 0.86C, so we can go with that.
One thing I did notice in the file, however, was that the optimal accuracy is +/- 5 per mille. The following link points out that the slope for the conversion from delta deuterium is 9 per mille per °C: http://cdiac.ornl.gov/trends/temp/domec/domec.html. So the 2 sigma error in the temperature estimate is at least +/- 1.1 °C just from the deuterium measurement alone.
All of this combined means that we can’t be sure at this time, based on the EPICA dataset alone, that the modern temperature has exceeded natural variation for the Holocene. So on this narrow point I admit that I was incorrect. Thankfully we have a lot more than one ice core dataset, and more than just ice core proxies. And those proxies all indicate that the sensitivity of the global climate to CO2 is somewhere between 2 and 4.5 °C per doubling of CO2. However, that’s a topic for a different post, and this discussion has wandered too far off topic already.
The fact remains that the optical properties of CO2 are such that adding CO2 to the atmosphere reduces the amount of heat that is re-radiated back into space. Your own MODTRAN fiddling show this, and my calculations show that it takes a tiny amount of additional CO2 to cause the observed increase in heat in the global ocean over the last 30 years. And it doesn’t change the fact that you’re wrong about your statement that the different heat capacities of the ocean vs. the atmosphere disprove industrial climate disruption (my preferred term). The only question I have now is whether you’ll acknowledge your error or not.
No one denies that but you can only trap 100% of something. With or w/o CO2 water vapor traps all the heat. Just add H2O to any MODTRAN calculation. CO2 is irrelevant. [SNIP]
ADMIN: Let’s just stop you right there. Here’s the applicable part of the comment policy that you’ve violated (emphasis added):
At this point it’s clear that you are not seeking meaningful engagement with Brian, and your refusal to address his arguments qualifies as bad faith behavior on your part. This is why your last two comments and most of this one have been deleted.
Commenting at S&R is a privilege, not a right. Exhibit good faith and we’ll moderate your comments through. Continue exhibiting bad faith and we won’t. Your choice.
Brian’s analysis that back IR can warm the oceans conveniently forgets that while back radiation is happening, there always is much more evaporation that is happening to cool the oceans. Heat goes from the oceans to the atmosphere via latent heat, NOT the other way around.
Alan, you responded to the original post, where I’m not talking at all about back radiation at all, just heat capacity. Which of the commenters and/or my responses are you responding to? Pointing it out by date would be appreciated so I can respond correctly.
Thanks.